MDR Development and Approval Step-by-step with Chimaera
At Chimaera, our service begins with the preparation of raw data and includes in-depth annotation services by medical experts. We guarantee the highest quality thanks to customized processes and, of course, ensure GDPR-compliant data processing. Based on our experience in numerous, successfully accomplished machine learning projects, we help you realize your project!
Your Strategic Start with Chimaera
AI projects may fail due to missing data, overly complicated annotation workflows, a wrong project setup – or just because you don‘t find a standard approach to solve your problem.
Plan with us right from the start to find out what you really need:
- Our experts assist in data planning, annotation and quality labeling
- Our experienced software engineers find a solution to your problem
- As a certified ISO 13485 company we support in regulatory affairs right from the start
- Together we choose the right AI product deployment
From prototype to an intelligent product
Trust in a competent partner for your customized AI project.
We offer quality-assured solutions in close cooperation across all steps of the development process.
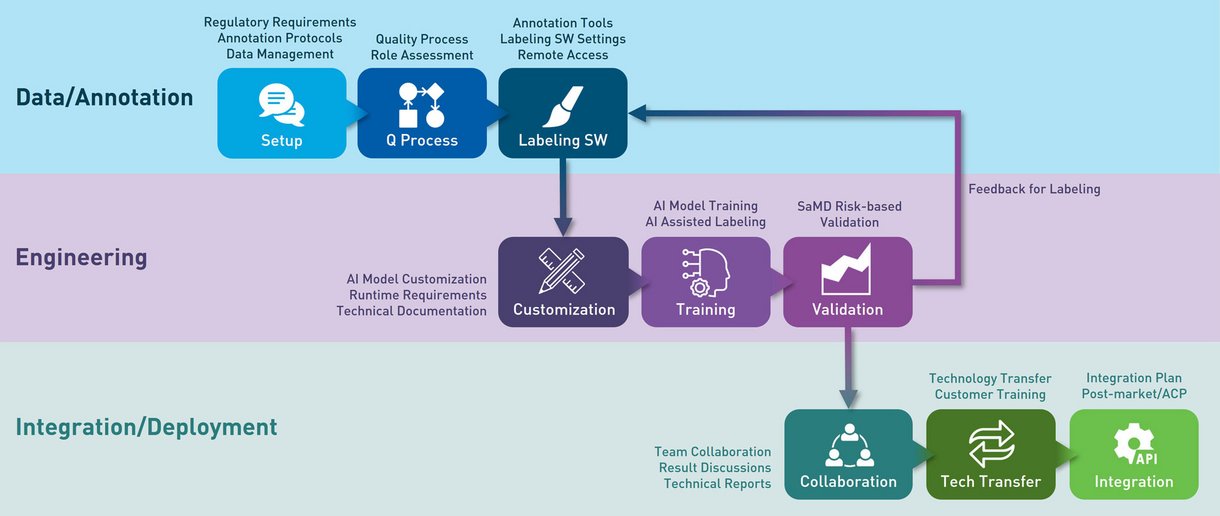
Annotation & Data Services
Our service starts with the preparation of raw data and includes in-depth annotation services by medical experts.
Our intelligent tools save you time while ensuring precision and consistency. Use tags, ROIs, or voxel masks for flexible annotation options and work effortlessly with DICOM and other formats. Our annotation tools are validated according to Computerized Systems Validation (CSV) requirements.
You have your own annotation team?
Perfect. Use our Annotation Tools on-premise or as a cloud service (AWS) to collaborate together globally.
Engineering Service
Our many years of experience and highly qualified machine learning experts make us the ideal choice.
We develop customized AI models that are specifically tailored to your product requirements and interfaces. Our in-house CUDA servers ensure maximum computing power to handle even complex tasks efficiently. We continuously optimize and update the models through incremental validation feedback and regular quality reports. All technical documentation is required for SaMD and risk-based validation procedures are implemented for SaMD.
Deployment, Integration and Technology Transfer
We do not deliver opaque "black boxes." Instead, we provide transparent source code, including trained models, training and validation scripts, and everything needed for on-premises retraining.
Our standardized AI backends (APIs) ensure flexibility, while structured data delivery with annotations, technology transfer, and customer training guarantee smooth deployment.
We plan and implement required Algorithm Change Protocols (ACP) to simplify post-market AI model changes.
Let's team together!
We offer close cooperation throughout the entire process, keep pricing and calculations transparent, and provide regular updates on the project. We'll also handle lifecycle management and provide comprehensive documentation. We ensure compliance with regulatory standards for data, labeling, development, and validation, reducing time-to-market and minimizing costs.
Chimaera is your all-in-one partner for medical image analysis, visualization, and MDR approval. We take a holistic approach, aligning technology with your business goals to get your cutting-edge products to market success.
