Gebündelte Kompetenz für KI/ML-Software als Medizinprodukt (SaMD) unter Einhaltung der MDR-Vorschriften und Software-Innovation.
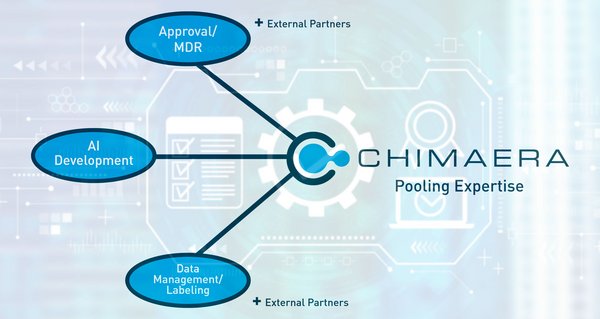
Die Expertise unseres Teams in den Bereichen KI-Entwicklung, Datenmanagement, Labelling und Regulatory Affairs stellt sicher, dass Ihr Projekt alle notwendigen Anforderungen für die Marktreife, wie z.B. MDR, erfüllt.
Compliance by Design
Um die Zeit bis zur Marktreife zu verkürzen und unnötige Kosten zu vermeiden, integrieren wir von Anfang an die richtigen Inputs und regulatorischen Standards für Daten, Kennzeichnung, Entwicklung und Validierung. Benannte Stellen (Notified Bodies) konzentrieren sich zunehmend auf Qualitätsanforderungen und die Auswahl von Datenpopulationen.
Unsere risikobasierte Datenselektion und unsere dokumentierten Labeling-Prozesse sorgen dafür, dass die richtigen Daten mit höchster Qualität ausgewählt werden. Ein KI-Modell ist nur so gut wie die Daten, mit denen es trainiert wurde!
Mit unserem erfahrenen Team und unserer vielseitigen Technologie decken wir ein breites Anwendungsspektrum ab: Klassifikation für die Diagnose, Clustering für die Datenanalyse, Segmentierung für die Erstellung von CAD-Modellen aus Bilddaten (CT, MR, DVT) und generative KI mit unbegrenzten Möglichkeiten.
We combine expertise in labeling, AI development, and regulatory affairs to bring your product to market safely and quickly.
Interested? Please, get in touch!
